Phosphorus fertilizers have many problems.
The extraction of rock phosphate and phosphorite is unsustainable, and with sources located in remote and geopolitically sensitive regions, extraction and supply is complicated.
Processing conventional fertilizer also requires the use of mineral acids, such as sulfuric acid, is energy intensive, and generates large amounts of hazardous by-product.
These issues combine to make a fertilizer that is expensive and environmentally damaging.
Yet, phosphate fertilizers are essential for feeding mankind. A 2017 studyfinding that as much as 60% of the planet’s arable land has reduced crop yields due to depleted phosphorus content in the soil. Often, such phosphorous deficiency occurs in some of the poorest countries in the world, where malnutrition is exacerbated by a lack of fertilizer supplies. For example, in India an estimated 49-45% of agricultural soils have low to medium phosphorus content.
One solution to this problem is the adoption of liquid biofertilizers sourced from phosphorus rich raw materials, such as bones and sewage sludge. Production of these fertilizers is cleaner and greener, using microorganisms that are naturally present in soil. One study also reported that they are highly efficient, yielding soluble phosphate content as high as 80% of raw material, which compares well with the 20-40% soluble content from extracted phosphorite.

However, sourcing phosphorus from sewage and bones can result in secondary environmental contamination. Research finding that they can contain a range unwanted and potentially toxic ingredients, such as pharmaceutical products, caffeine, synthetic phenolic compounds, siloxanes, per fluorinated compounds, and toxic hydrocarbon. Removing these contaminants to allow for use in fertilizer production is expensive, requiring chemical or membrane filtration and/or heating to extreme temperatures.
To solve all these issues, a team of researchers have employed nanotechnology and a biosynthetic approach for a novel way to make the phosphate fertilizer nanohydroxyapatite (nHAP).
The creation of nanohydroxyapatite (nHAP) is not new, with several different production processes, such as wet chemical deposition, sol-gel, hydrothermal, and biomimetic routes. However, all of these methods require the overt use of numerous chemicals to achieve controlled synthesis, with the resulting nanomaterials having a high level of toxicity.
By employing a biological approach, the new nanofertilizer process has a much lower environmental impact, and can, the study says, “… [also] control the structure, orientation and phase, nano-structural topography of inorganic crystals, called bio-mineralization.”
The method can also control the shape and size of the nanoparticles by varying factors such as temperature, pH, stirring rate, and sintering temperature during and after biosynthesis.
Specifically, this novel nanofertilizer employs bacillus licheniformis for biosynthesis of a nanostructured hydroxyapatite powder by using calcium and phosphorous precursors.
The study, now published in the journal Nature, describing how, “The major mechanism underlying nanoparticle synthesis involved phosphorus (P) solubilization by production of gluconic acid through the pqq gene cluster present in B. licheniformis. This solubilized P then combined with Ca in a sol-gel manner to yield nHAP.”
The outcome is a powder of crystalline particles with a size range of 25 – 35 nm (dependent on phosphate concentrations, which the report notes as, “… 2%, 5%, 10% and 20% w/v of potassium dihydrogen orthophosphate monobasic (K2HPO4).”
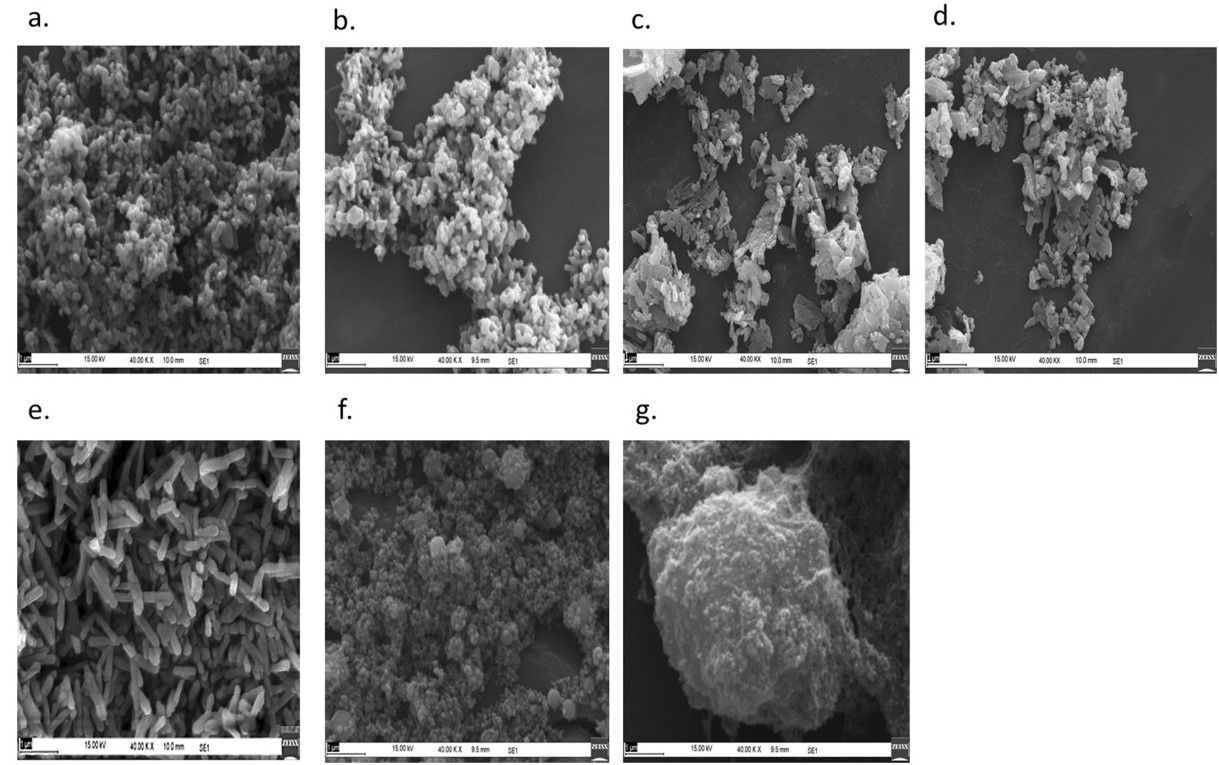
Adding that tests have shown, “… no adverse effect on plant growth-promoting bacteria.”
The ultimate result is a nanofertilizer with a size, shape, and structure that makes phosphorus highly available for crops.
While the team at the feasibility of industrial nanofertilizer production, they are confident that the relative simplicity of the technique will ensure a straightforward upscale.
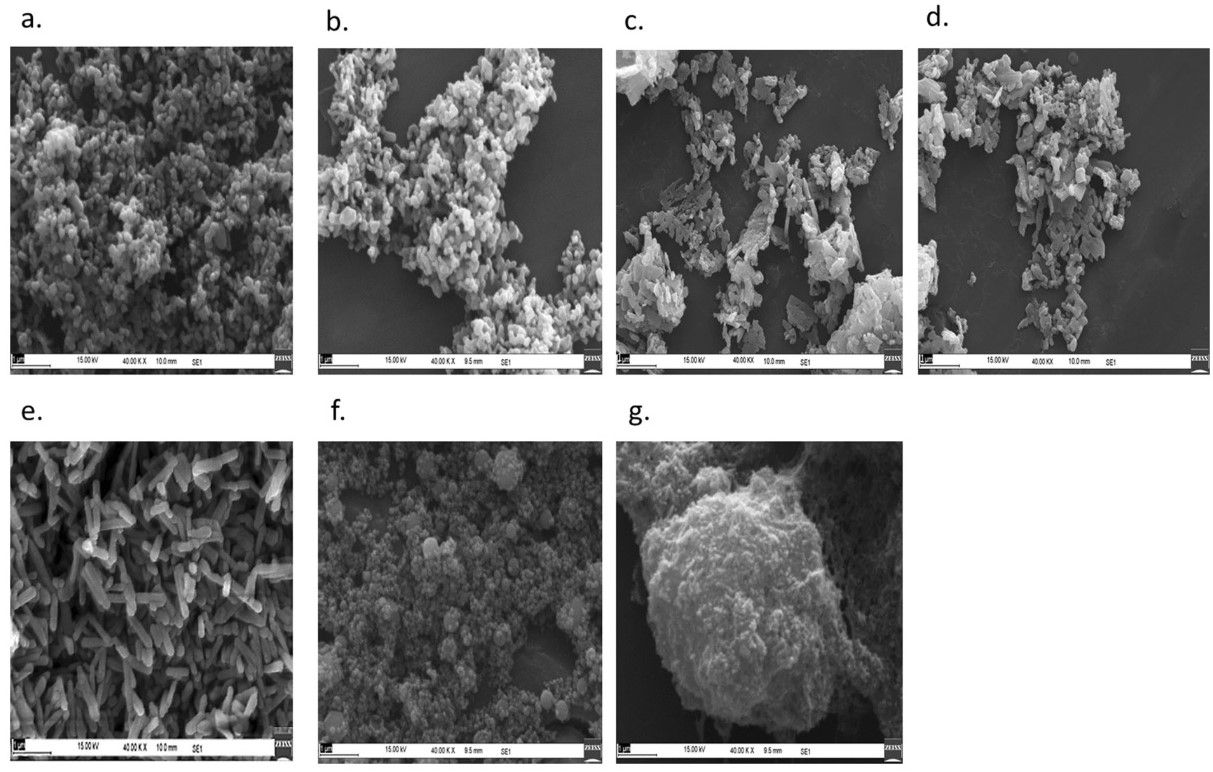
Furthermore, while the challenge of nurturing sufficient microorganism growth remains, the process allows for the safe handling of the bacteria, leading to cost-effective manufacture.

With consumers, politicians, and farmers all questioning the sustainability of modern fertilizer practices, the continued use of bulk rock phosphate supplies that leads to soil toxification and the eutrophication of rivers, lakes, and oceans, points to a likely increase in the adoption of nanofertilizer.
Given its high efficiency and effectiveness and the need to replenish phosphate availability in so much of the world’s farmland, the discovery of a cost-effective process for nHAP production is sure to be a positive move.
Photo credit: Nature, Aces, fertilizerproduction, Ifad, & drylandsystems
