Anyone who has ever used an electronic device knows how quickly and easily they get warm. While in summer a hot tablet or mobile phone may only be an inconvenience, in extreme conditions the heat can cause malfunctions, require extra power usage for fans in laptops, and in rare cases may even cause lithium batteries to explode.
Current methods of avoiding these problems revolve around building more efficient cooling devices, however these require more power and are typically too large and heavy for handheld devices. In these cases, layers of plastic, inert glass, or even air pockets are used as insulation to stop heat-generating components, such as microprocessors, from damaging other parts or causing discomfort for the user.
However, by applying nanoscale materials, a team from Stanford University have created a heat shield which is just 10 atoms thick. This could make future insulating devices as much as 50,000 times thinner than the materials currently used in phones and tablets.
To make such a breakthrough requires a full understanding of how and why electronic devices heat up, which, as the Stanford University website explains, is not how and why you would expect.
“The heat we feel from smartphones or laptops is actually an inaudible form of high-frequency sound,” reports the Stanford press release. “If that seems crazy, consider the underlying physics. Electricity flows through wires as a stream of electrons. As these electrons move, they collide with the atoms of the materials through which they pass. With each such collision an electron causes an atom to vibrate, and the more current flows, the more collisions occur, until electrons are beating on atoms like so many hammers on so many bells - except that this cacophony of vibrations moves through the solid material at frequencies far above the threshold of hearing, generating energy that we feel as heat.”
Knowing that it was in fact sound that was making things hot, inspired the researchers to think how we already insulate against noise. For example, soundproof booths in radio stations use thick glass to block exterior voices, however the thickness and weight of glass is impractical for ultrathin electronic devices.
Instead, the team began to think about how homes use double or triple glazing as insulation.
We adapted that idea by creating an insulator that used several layers of atomically thin materials instead of a thick mass of glass,” explains Sam Vaziri, a postdoctoral scholar and the lead author on the paper.
The key to the new nanoscale insulation was stacking atomically thin layers one on top of another like sheets of paper. In doing so, the researchers showed that this nanomaterial provided as much heat insulation as a sheet of glass 100 times thicker.
As the industry journal Nano-Tech Now reports, “The Stanford team used a layer of graphene and three other sheet-like materials - each three atoms thick - to create a four-layered insulator just 10 atoms deep.” Adding that, “Despite its thinness, the insulator is effective because the atomic heat vibrations are dampened and lose much of their energy as they pass through each layer.”
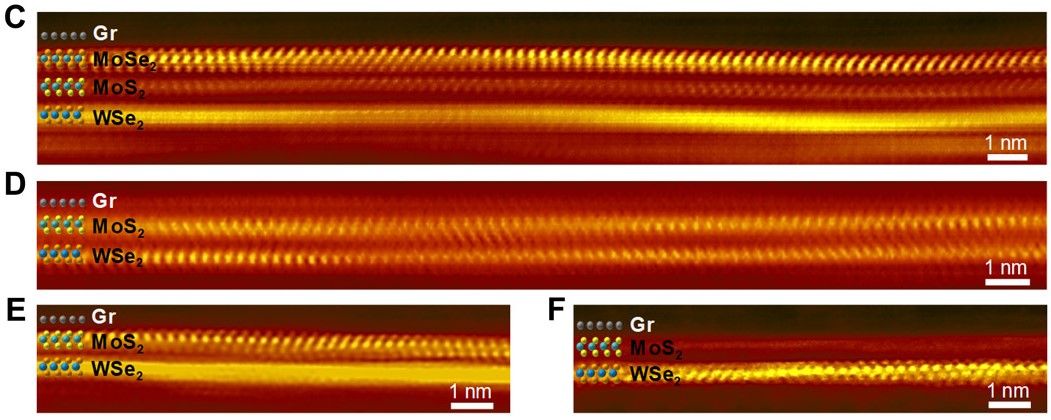
The team have now published their results in the journal Science Advances, where they state how they can, “… demonstrate unusually high thermal isolation across ultrathin heterostructures, achieved by layering atomically thin two-dimensional (2D) materials.” This they achieved through, “… artificial stacks of monolayer graphene, MoS2 [molybdenum disulphide], and WSe2 [tungsten diselenide] with thermal resistance greater than 100 times thicker SiO2 [the silicon dioxide used to make glass] and effective thermal conductivity lower than air at room temperature.”
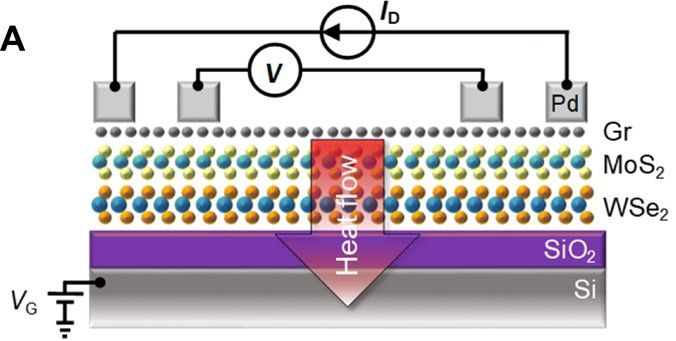
The next challenge is taking the nanotechnology and applying it to the manufacture of electronic devices. However, the team are confident that a way can be found to deposit the atom-thin layers onto electronic components, possibly via a spray.
But this breakthrough is more than just about designing an ultrathin, lightweight heat shield, it is about rethinking how vibrational energy inside materials could be used to control light, heat, and electricity. By seeing the heat inside solid objects as a form of sound, the field of phononics is taking shape.
“We're looking at the heat in electronic devices in an entirely new way,” says Eric Pop, a professor of electrical engineering and the study’s senior author. “As engineers, we know quite a lot about how to control electricity, and we're getting better with light. But we're just starting to understand how to manipulate the high-frequency sound that manifests itself as heat at the atomic scale.”
One clear example of this, has been found by researchers from MIT, who have developed a battery free sensor that is able to measure, send and receive data without any previously practical energy source.
The system makes use of two key phenomena; the ‘piezoelectric effect’, which turns vibrations in a material into an electrical charge, and ‘backscatter’, a communications technique which transmits data by bouncing modulated radio signals off a tag and back to a reader.

As the MIT Review outlines, “In the researchers’ system, a transmitter sends acoustic waves through water toward a piezoelectric sensor that has stored data. When the wave hits the sensor, the material vibrates and stores the resulting electrical charge. Then the sensor uses the stored energy to reflect a wave back to a receiver — or it doesn’t reflect one at all. Alternating between reflection in that way corresponds to the bits in the transmitted data: For a reflected wave, the receiver decodes a 1; for no reflected wave, the receiver decodes a 0.”
“Once you have a way to transmit 1s and 0s, you can send any information,” says the study’s co-author Fadel Adib, an assistant professor in the MIT Media Lab and the Department of Electrical Engineering and Computer Science. “Basically, we can communicate with underwater sensors based solely on the incoming sound signals whose energy we are harvesting.”
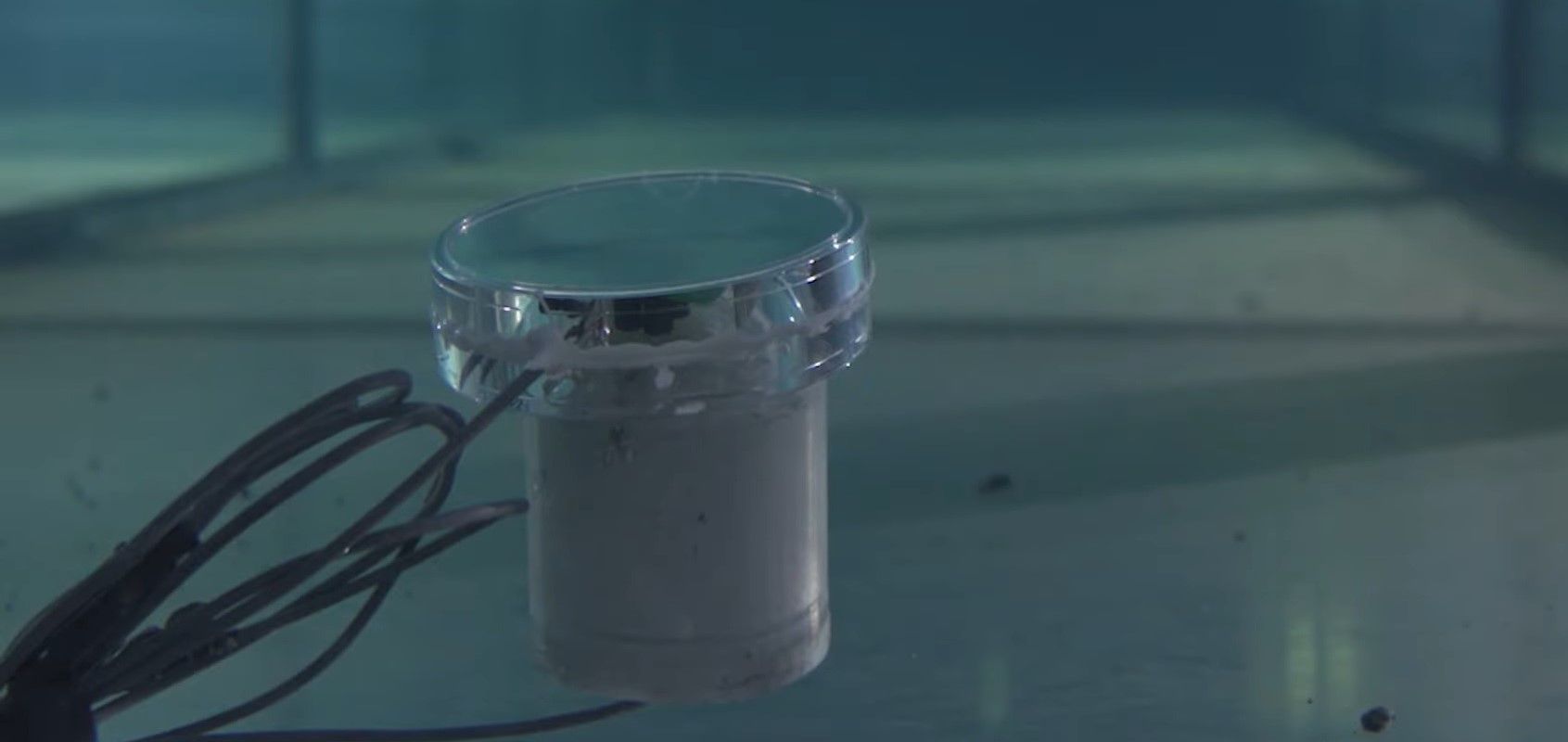
At present, the technology is being seen as a way to develop sensors for remote and extreme locations, such as on distant planets or on the seabed, where solar panels would be useless.
“How can you put a sensor under the water on Titan that lasts for long periods of time in a place that’s difficult to get energy?” asks Adib. “Sensors that communicate without a battery open up possibilities for sensing in extreme environments.”
While this is the current goal, in the future it is thought that a better understanding of using sound waves inside a material could be a route to powering all manner of devices. Graphene sheets, carbon nanotubes, nanowires, and other nanoscale materials are really beginning to show how we can rethink our world.
While today devices powered by the vibrations in a material may be close to science fiction, ultrathin layers of nanomaterial for use as a mobile phone heat shield could be developed in a few years.
In fact, understanding nanomaterials, atomic-scale structures, and their manufacture and supply will be the key to Industry 5.0.
As Richard Smalley, winner of the 1996 Nobel prize for Physics for his discovery of nanoparticle buckyballs notes, “When you control atoms, you control just about everything.”
Photo credit: Science Advances, MIT, Express, Bayviewwindows, & Slideserve
