As electronic devices get smaller and smaller, demand for smaller batteries is getting bigger and bigger. In fact, the growing sector in today’s battery industry is for micro-batteries.
This has fuelled a large amount of research into new raw materials and designs for cell structures, electrodes, electrolytes, and new production processes.
In the past, limited battery technology meant that electronic devices had to be designed around the size and shape of the battery. Even today, removing the back of most watches or smartphones shows how much space and weight is taken up on energy storage.
Nanomaterials for the World’s Smallest Batteries.
All that is now changing, as the onset of nanomaterials and 3D printing is enabling the construction of smaller more efficient batteries or batteries that can be printed into any shape desired. In some cases, this means energy storage devices so small, that they cannot be seen with the naked eye.
As Rice University reported in 2016, “Researchers have built a battery that's six times thinner than a bacterium. At 150 nanometres wide, the nano battery is more than 60,000 times smaller than a AAA battery. The microscopic power pack could be used to run all sorts of minuscule electronic devices, including sensors that spy on single cells.”
However, the limited power capabilities combined with the challenges in handling and manufacturing make such small batteries impractical except for a few specialised applications.
Instead, most research is focused on packing together large amounts of nanomaterial to capture the combined force of their electricity holding properties.
Carbon Nanotubes for Batteries.
One such study, by Peter Burke, a professor of electrical engineering at the University of California, Irvine focused on improving an electrolyte’s ability to store energy with a combination of carbon nanotubes and saltwater.
As the university press release reports, “Instead of experimenting with metal, Burke and his collaborators used a 0.1-millimeter-square tangle of one-dimensional, atomic-sized wires called nanotubes. Each nanotube is only a few atoms wide and because of their extremely small size, have different quantum mechanical behavior than regular metals.”
“As things get smaller, their quantum properties become very important,” Burke explains. “When they’re big, materials behave more or less classically. The ability to store a charge is very, very different in a nanotube because of the quantum mechanical effects that occur at the atomic scale.”
By standing as many as 10,000 nanotubes (each measuring only one or two nanometres in diameter) on end the tubes form a spikey, ridged array with a high surface area.
“It’s like a bunch of knives, all sticking up,” describes Burke.
This larger surface area increases capacitance. Additionally, the saltwater atoms are of a similar size to the nanotubes which enhances the relationship between the charge and the nanotubes. The result, according to Burke, it that, “They store a lot more charge.”
Nanowires for Batteries.
Other research conducted at Irvine has focused on using nanowires, tiny strips of material which can be a thousand times thinner that a human hair. While exceptionally thin, their large surface area and high electrical conductivity are superb properties for battery raw material.
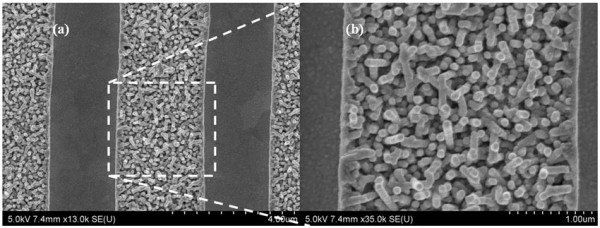
Knowing this, “The Irvine researchers coated the gold nanowires in manganese dioxide shells encased in an electrolyte of gel. The result is no loss of power even when recharged 200,000 times over three months.”
3D Printing Carbon Nanotubes for Batteries.
Further research is looking into the advantages to be gained by 3D printing carbon nanotubes as a raw material for batteries. As a recent study, published in the journal Advanced Functional Materials, concludes, “It is expected that with the continuous development of printing techniques and materials, 3D‐printed batteries with long‐term durability, favourable safety as well as high energy and power density will eventually be widely used in many fields.”
This is an issue also highlighted by the nanotech journal, Nanowerk, which reports that, “Carbon nanomaterials are another promising material group for the electrode materials used in 3D-printed batteries. Reduced graphene oxide and graphene, for instance, have been used to 3D print ultrafast supercapacitors.”
The popularity in using carbon nanotubes and carbon nanofibers for printing inks is due to their high mechanical strength, large surface area, high chemical stability, and excellent electrical and thermal properties.
These properties give nanotubes plenty of potential for use across the battery industry and make them and other nanomaterials likely to become a mainstay raw material for future batteries both large and small.
Photo credit: Nanowerk, Pocketlint, Cnet, MDTCreative, & Engadget
