Since their discovery in 2004, researchers have been exploring the astounding properties of nanomaterial sheets, such as graphene, borophene, germanene, silicene, stanene, plumbene, and a growing list of other perfectly thin materials.
As they are only an atom or so thick, these 2D materials operate in a very different way to the same substance in a 3D form. This gives them huge potential in every sector of the economy, with improved electrical conductivity, strength, flexibility, ultra-low weight, and other much sought-after properties.
So, why are 2D materials not in much greater use?
The key reason is that they are still relatively slow and expensive to produce, with numerous factors needing to be considered to create high-quality 2D nanomaterials. Pressure, flow rate, temperature, and precursor type, as well as the raw material used as a substrate must all be considered before chemical vapor deposition (CVD) synthesis can begin. Even then, there are extreme limitations on how much of the process can be controlled. These restrictions on what can and cannot be made means that only a few types of 2D materials can be produced into large area, high quality films.
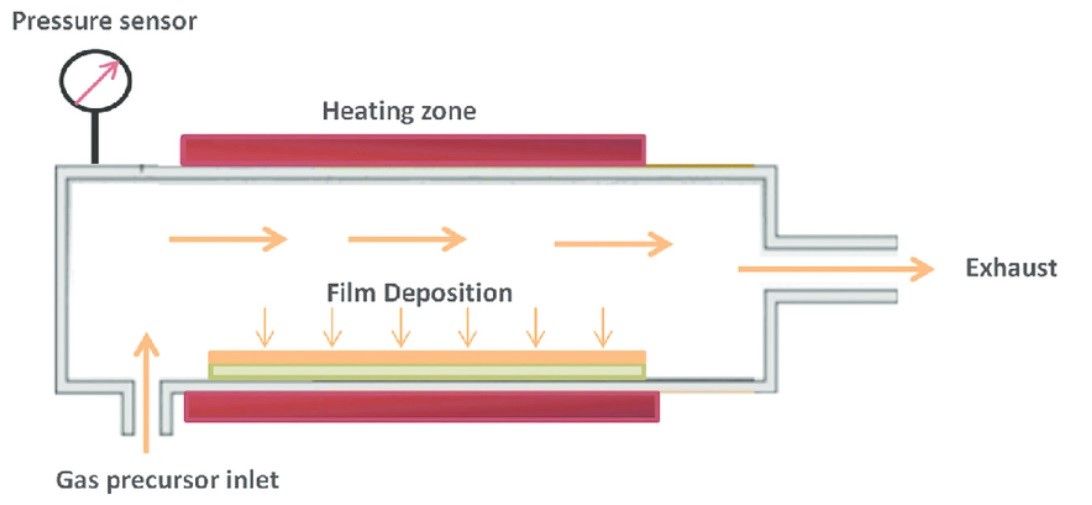
As a result, graphene sheets are often quite small, and their high cost limits them to applications in research or other advanced fields. For example, miniscule 2D sheets can be used as heat sinks in hi-spec electronics, in batteries and sensors, or similar advanced electronics in aerospace and mobile devices.
But now 2D nanomaterials may be about to break into mass production, a multinational research team has just discovered that fluorine can dramatically decrease synthesis time. This potentially paves the way for cheaper and more industrialised manufacturing of 2D nanomaterials.
Most of the scientists were based at the Center for Multidimensional Carbon Materials (CMCM) at the Ulsan National Institute of Science and Technology (UNIST) in South Korea, but worked in cooperation with Chinese nanospecialists in growing three types of 2D material; graphene, h-BN, and WS2.
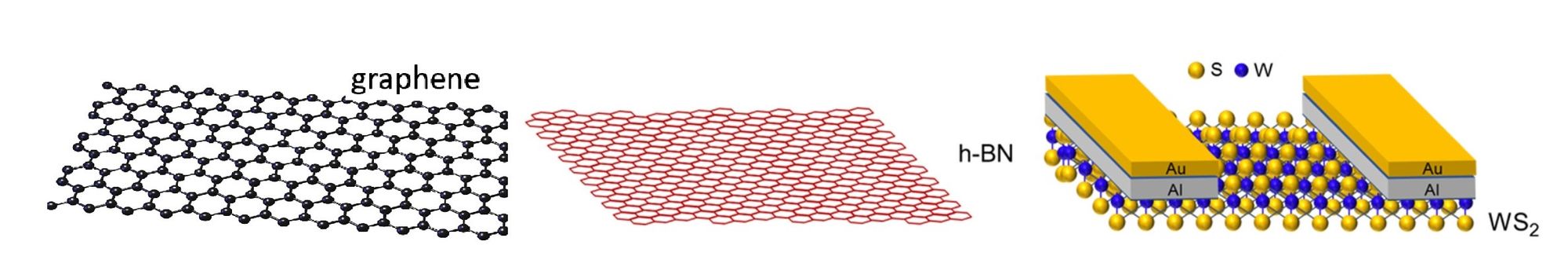
The study focused on the element fluorine, because, as the Institute for Basic Science explains, “… it requires only one electron to attain high stability. It also has seven electrons in the outermost orbit of an atom, the distance at which these valence electrons reside is the minimum compared with other elements. This means the valence electrons of fluorine are bound to the atom more strongly than any other atom making fluorine the most active element in the periodic table.”
Understanding these chemical advantages led the study’s lead-author Prof. Feng Ding, to question why the nanomaterial industry continues to use other active gases such as hydrogen or oxygen.
“Why not use the most active element, fluorine?” he asked. “The highest electronegativity allows fluorine to form bonds with nearly all the atoms in the periodic table, so it is expected to change the reaction routes of many chemical processes.”
The reason is that fluorine becomes highly toxic in the reactor of the CVD process. However, Prof. Ding and his team found a solution to this toxicity by limiting the supply of fluorine, so that only a minimum amount is used.
This worked by placing the fluorine substrate below a copper foil with a very small gap between. At the high temperatures involved the fluorine radicals are released from the fluoride surface and trapped in the narrow gap, limiting the toxicity.
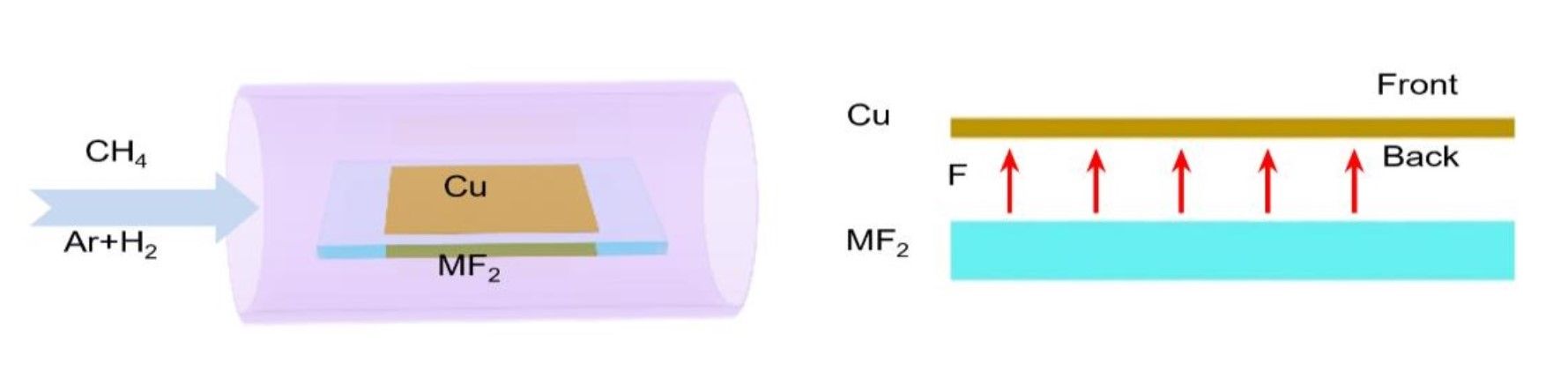
The results were surprising and allowed for a record graphene growth rate of 12 mm per minute. This far surpasses standard techniques for manufacturing 2D nanomaterials, where an expected synthesis time for a 10 cm2 graphene sheet is 10 minutes. By using fluorine, the same sized sheet can be produced in 3 minutes.
As the online journal Science Daily, explains, “The introduction of local fluorine entirely changes the methane decomposition route,” the report notes. “As the fluorine released from the metal fluoride surface easily reacts with methane gas, there will be a sufficient amount of CH3F or CH2F2 molecules in the gap between Cu and BaF2 substrates. These molecules could decompose on a Cu surface much more easily than CH4 does. In other words, they feed the graphene growth better by supplying more active carbon radicals (i.e. CH3, CH2, CH and C).”
Continuing their work, the researchers discovered that this process of limiting fluorine supply also sped up the growth of other 2D materials, such as h-BN and WS2.
As a result, the researchers are confident in the impact of their results on the nanotechnology industry.
“We envision that this local fluorine supply will readily facilitate fast growth of broad 2D materials,” notes Prof. Ding. “Or enable the growth of new 2D materials which are difficult to be realized by other methods.”
Furthermore, it is not only fluoride that has the chemistry for the accelerated growth of nanomaterials. Other chemical substrates such as sulphides, selenides, bromides, or chlorides may also produce a similar effect if their supply is controlled accurately. This will create a range of scientific possibilities to aid the expansion of 2D nano-sheeting throughout industry.
Photo credit: IBS, Researchgate, Laboratoryjournal, Researchgate, Singularityhub, & Thegraphenecouncil
