In a world demanding ever-higher quality energy storage, flow batteries have great potential.
Moreover, they also have the ability to rejuvenate renewable energy sources that up until now have been limited by an inability to efficiently store electricity. For example, solar panels provide power when the sun shines, but household energy demand rises at night, when it is colder and dark.
Flow batteries, as David L. Chandler of the MIT news office explains, “…foster renewable energy by providing inexpensive storage for generated electricity.”
While the engineering is technical, the concept is relatively simple. Instead of solid electrodes, flow batteries contain a slurry of tiny particles suspended in liquid. This allows battery capacity to be easily increased by merely adding bigger tanks. However, they currently have one major flaw; they are limited by flow rates and so lack efficiency.
That problem may now have been solved as a research team from MIT have published a study announcing a nanocoating that may provide some necessary slipperiness.
The lead-author of the study, Kripa Varanasi, a professor of mechanical engineering, is already well known for his research in developing a nanocoating that prevents semi-solids from sticking in containers. Such was that research’s success that a company, LiquiGlide, was created to commercialise sales to manufacturers of shower gel and ketchup.

By applying nanoscale lubricant-impregnated surfaces (LIS) to the flow battery contents the slurry is able to move more freely, dramatically increasing efficiency.
As Chandler illustrates, “These coatings could resolve a conundrum that flow battery designers have faced, because they needed to add carbon to the slurry material to improve its electrical conductivity, but the carbon also made the slurry much thicker and interfered with its movement.” This led to a situation described by Varanasi as having, “a flow battery that couldn’t flow.”
Together with MIT professors Gareth McKinley and Yet-Ming Chiang, as well as Xinwei Chen and Brian Solomon PhD, Varanasi has used the nanocoating to find, “… up to 86% mechanical power savings at low flow rates for lubricant-impregnated surfaces (LIS) compared to conventional surfaces for a lithium polysulfide flow electrode in a half-cell flow battery configuration.”
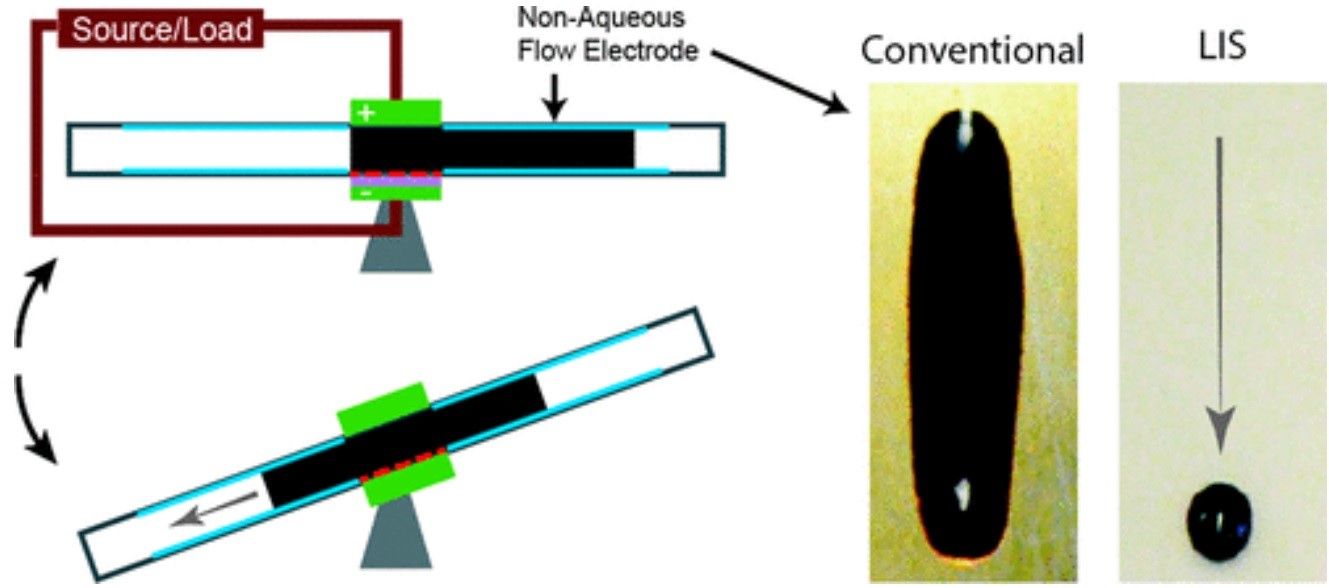
Publishing their findings in the journal Applied Energy Materials, they describe how, “Today, most redox flow batteries are based on aqueous solutions with low cell voltages and low energy densities that lead to significant costs from hardware and balance-of-plant. Nonaqueous electrochemical couples offer higher cell voltages and higher energy densities and can reduce system-level costs but tend toward higher viscosities and can exhibit non-Newtonian rheology that increases the power required to drive flow.”
Adding that, “The measured specific charge capacity of ∼800 mAh/(g·S) is a 4-fold increase over previous work.”
“Previously flow batteries had a trade-off in that as you add more carbon particles the slurry becomes more conductive, but it also becomes thicker and much more challenging to flow,” says Brian Solomon PhD, a co-author of the study. “Using slippery surfaces lets us have the best of both worlds by allowing flow of thick, yield-stress slurries.”
Due to their previous inefficiencies flow batteries have few practical applications in national power grids. However, proof of their potential lies in China, where the vanadium flow battery array in Dalian (pictured) can store 200MW/800MWh.
Once construction has been completed, the battery will coordinate with other energy storage facilities in the area to capture wind power that at present cannot be held in reserve. This means that at present nearby wind turbines have been forced to lower electricity production as supply sometimes outstrips demand.
The Dalian flow battery hopes to improve this situation.
As the industry journal Electrek, notes, “The battery’s purpose is to provide power during peak hours of demand, to enhance grid stability and deliver juice during black-start conditions in case of emergency. The system is expected to peak-shave about 8% of Dalian’s expected load when it comes online in 2020.”
Not only has the discovery of a new slippery nanocoating made flow batteries a more practical solution, they have also shown how nanotechnology can open doors to new areas of exploration.
As Soloman explains, “Apart from fabricating a flow battery device which incorporates the slippery surfaces, we also laid out design criteria for their electrochemical, chemical, and thermodynamic stability. Engineering surfaces for a flow battery opens up an entirely new branch of applications that can help meet future energy storage demand.”
Photo credit: MIT, Applied Energy Materials, ScienceMag, IEA, & Electrek
