One of today’s most pressing issues is the problem of plastic waste. A problem that may now have been solved by using nanoparticles as a catalyst to convert plastics to useful products such as engine oil, cosmetics, and detergents.
Each year, nearly 400 million tons of plastic is produced worldwide, a number that is predicted to increase fourfold in the next 30 years. With more than 75% of plastic made for single use products, such as plastic bags and water bottles, it is inevitable that some of this plastic will not be disposed of properly.
While efforts are being made by communities to increase proper disposal of plastic so that it may be recycled, manufacturers are also looking for ways to replace plastic. Although, in many situations, such as medical devices and food packaging, plastic is simply the best material available.

However, current technology is limited in the type of plastic it can economically recycle and what the plastic waste can be recycled into. Typically, this means that high quality plastic is converted into a plastic product of slightly less value and worse quality, which can then be recycled into an even less valuable, lower quality polymer, until eventually the plastic is of such poor quality that it can only be burnt or buried in landfill.
To avoid this ever-decreasing circle of plastic recycling requires a breakthrough method for rejuvenating the chemicals and raw materials in plastic.
“There are certainly things we can do as a society to reduce consumption of plastics in some cases, but there will always be instances where plastics are difficult to replace,” says Aaron Sadow, a scientist and team leader of the nanoparticle breakthrough. “So, we really want to see what we can do to find value in the waste.”
As Max Delferro, group leader of the Catalysis Science Program in the Chemical Sciences and Engineering division at the U.S. Department of Energy’s (DOE’s) Argonne National Laboratory, notes, “We asked ourselves the question, ‘What can we do about it [plastic waste] in terms of chemical recycling?’. So, we assembled a talented team with DOE’s Ames Laboratory and researchers from top universities to provide a solution.”

The team was made up of academics from esteemed universities such as Cornell, Northwestern, University of South Carolina, and University of California, Santa Barbara.
Much of the problem lay in the fact that many plastics simply do not degrade easily because of their strong carbon-carbon bonds. Polyethylene and other polyolefins, in particular, are very difficult to catalytically deconstruct, yet the team believed that only by using efficient catalysts would economic recycling of lower grade polymers be achieved.
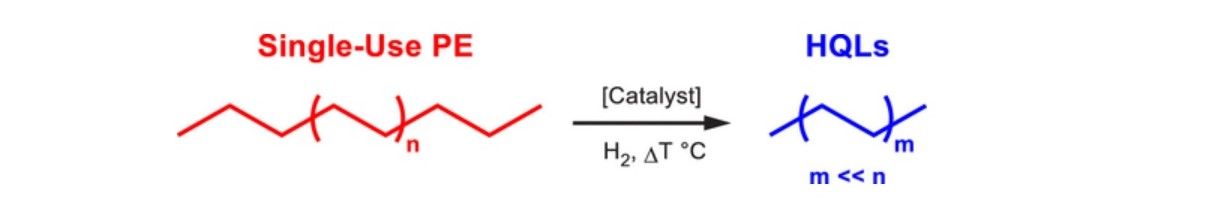
The solution may now have been found in the form a catalyst made up of platinum nanoparticles and perovskite nanocuboids that can breakdown plastics into a financially viable form.
“We sought to recoup the high energy that holds those bonds together by catalytically converting the polyethylene molecules into value-added commercial products,” says Delferro.
As the scientific journal, Cosmos Magazine, notes, “Under moderate pressure and temperature, the researchers say, the catalyst cleaves plastic’s carbon-carbon bond to produce high-quality liquid hydrocarbons. This is in stark contrast to commercially available catalysts, which generate lower quality products with many short hydrocarbons, limiting the products’ usefulness.”
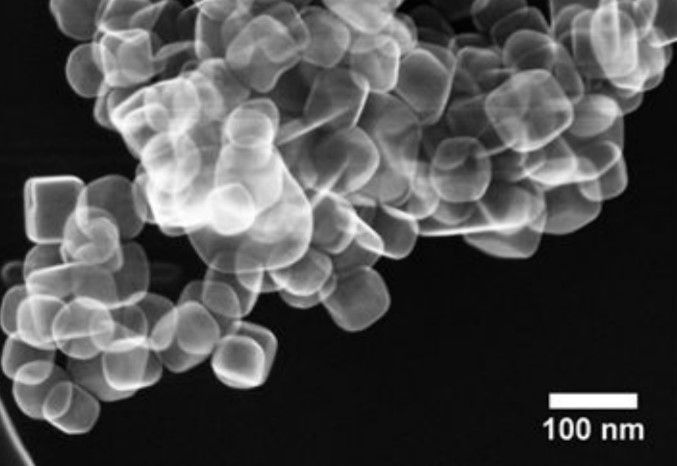
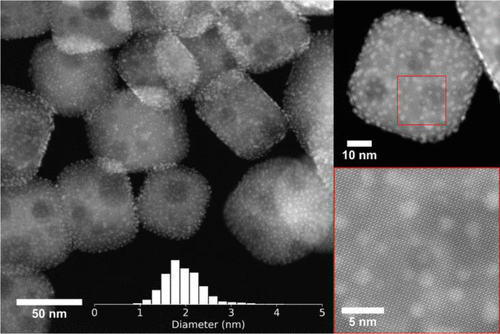
One of the bigger hurdles in creating the catalyst, was in placing the platinum nanoparticles - each measuring only 2 nanometres in size – on top of the perovskite nanocuboids which themselves only measure about a hundred nanometres. This was achieved through the cutting-edge method of atomic layer deposition, developed by researchers from Argonne and Northwestern for the precise manipulation of such miniscule objects.

Perovskite was chosen due to its stability under the extreme pressure and temperature required for catalysis, as well as for its proven energy conversion properties.
Once the catalyst had been made, testing could begin, starting with a high-quality polymer - research-grade polyethylene – one of the most common components in everyday plastic. As the nanotechnology journal Nanowerk, explains, “Under the appropriate reaction conditions, the catalyst converted the polyethylene into a high-quality liquid product in high yield. Next, they tested the catalyst with a commercial plastic bag. Remarkably, the catalyst was just as effective with the plastic bag as pure polyethylene, and it produced far less of the light hydrocarbons (methane, ethane, etc.) generated by pyrolysis - a process that involves heating at high temperatures - or use of a conventional catalyst consisting of platinum nanoparticles on an alumina substrate.”
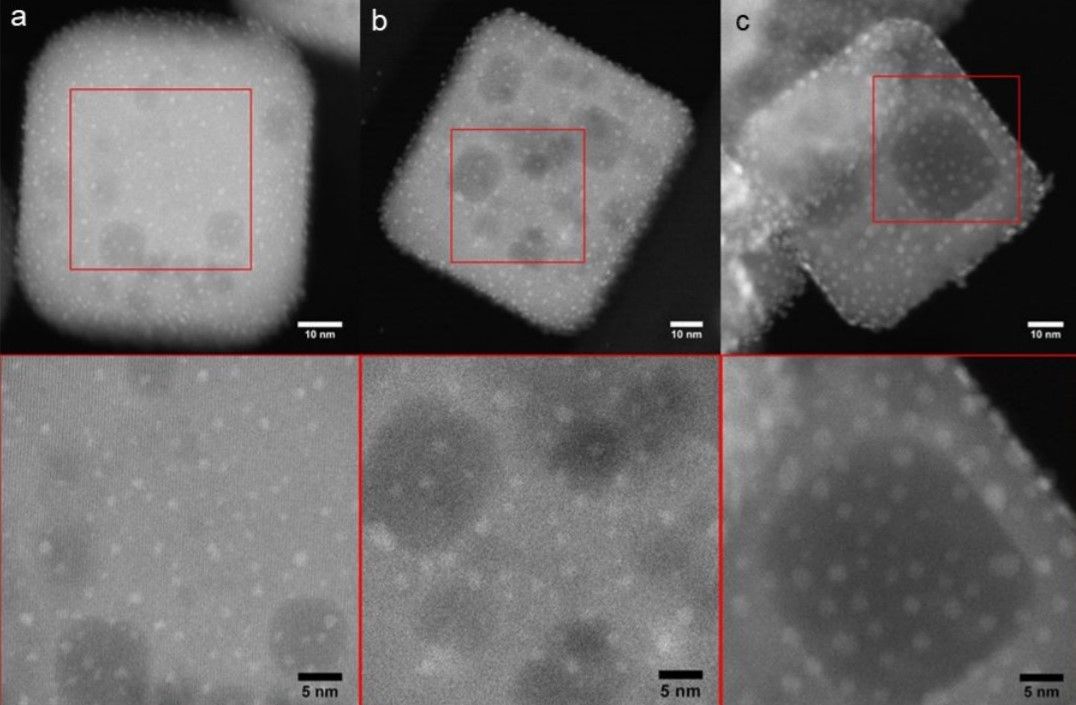
Further tests revealed that two key features were being applied by the nanoparticle raw materials to make an effective catalyst. As the Nanowerk report, describes, “One is the thermal stability of the platinum nanoparticles on the larger perovskite particles, due to the close geometric match between the cubic shapes of the nanoparticles and the support. The other is the non-porosity of the perovskite material, which promotes the catalytic reaction.”
The results have greatly impressed the team and others in the scientific community.
As Prof. Kenneth R. Poeppelmeier, the director of Northwestern’s Center for Catalysis and Surface Science and a member of the Program on Plastics, Ecosystems, and Public Health exclaimed, “Our team is delighted to have discovered this new technology that will help us get ahead of the mounting issue of plastic waste accumulation.” Adding that, “Our findings have broad implications for developing a future in which we can continue to benefit from plastic materials, but do so in a way that is sustainable and less harmful to the environment and potentially human health.”
As the study, now published in the journal ACS Central Science, outlines, “…inefficient recycling and extremely slow environmental degradation of plastics are causing increasing concern about their widespread use. After a single use, many of these materials are currently treated as waste, underutilizing their inherent chemical and energy value. In this study, energy-rich polyethylene (PE) macromolecules are catalytically transformed into value-added products by hydrogenolysis using well-dispersed Pt nanoparticles (NPs) supported on SrTiO3 perovskite nanocuboids by atomic layer deposition. Pt/SrTiO3 completely converts PE or a single-use plastic bag into high-quality liquid products, such as lubricants and waxes, characterized by a narrow distribution of oligomeric chains, at 170 psi H2 and 300 °C under solvent-free conditions for reaction durations up to 96 hours.”
“Plastic pollution is obviously a big problem,” concludes Delferro. “No one wants to go on vacation at the beach only to find plastics strewn about everywhere. While our catalyst still needs further development, our results look very promising for contributing to the national laboratories’ mission of solving some of the world’s most complex problems.”
Photo credit: ACS Central Science, Azocleantech, & ANL
