A new and comprehensive study of energy storage methods has found that the way to saving the planet is through nanomaterials.
While climate scientists have repeatedly proven the damage that modern industry and consumerism is doing to the environment, making a change from burning fossil fuels is a daunting task. However, the new report states that the path away from oil and gas is already known to us. What is needed is 21st century battery technology to enable the practical generation of energy from renewable sources.
Of all the actions that cause climate change, it is the way that energy is used which is most harmful. There is plenty of energy all around us in the form of wind, wave, and solar power. There is also sufficient technology available to capture enough of this power to meet all our needs and more. The problem is that these energy sources are unreliable, as no one knows when the wind will blow, when the waves will flow, and when the sun will shine.
What we lack is sufficient technology to store electricity until it is needed.
As Yury Gogotsi, PhD, Distinguished University and Bach professor at Drexel University's College of Engineering and the paper’s lead author, highlights, “The better we become at harvesting and storing energy, the more we'll be able to use renewable energy sources that are intermittent in nature.”
If technologies could be developed to better store the power from these energy sources, then the battle against climate change would be won. The clearest route to developing improved batteries is through the use of nanomaterials.
“Most of the biggest problems facing the push for sustainability can all be tied back to the need for better energy storage,” says Gogotsi. “Whether it's a wider use of renewable energy sources, stabilizing the electric grid, managing the energy demands of our ubiquitous smart and connected technology or transitioning our transportation toward electricity - the question we face is how to improve the technology of storing and disbursing energy. After decades of research and development, the answer to that question may be offered by nanomaterials.”
The logjam between renewable energy capture and point of consumption has long been known and has been the driving force behind a concerted effort in the scientific community to improve battery performance. Advances over the past decade in the way that smartphones, electric cars, and laptops absorb and store electricity are evidence of what is possible.
The high surface-to-volume ratio and short diffusion pathways of many nanomaterials can provide both high energy and power density. Furthermore, the compatibility of nanomaterials with modern manufacturing techniques, such as printing, spray coating, and roll-to-roll assembly, allows for the design and realization of wearable, flexible, and foldable energy storage devices.
At the heart of this technology has been the use of nanomaterials. As Gogotsi explains, “Many of our greatest achievements in energy storage in recent years are thanks to the integration of nanomaterials. Lithium-ion batteries already use carbon nanotubes as conductive additives in battery electrodes to make them charge faster and last longer. And an increasing number of batteries use nano-silicon particles in their anodes for increasing the amount of energy stored. The introduction of nanomaterials is a gradual process and we will see more and more nanoscale materials inside the batteries in the future.”
While for years, battery design was focused purely on finding progressively better energy materials and combining them to store more electrons, recently, research has turned to examining how to improve these devices for the better transmission of electricity and looking at how power storage works at an atomic level.
As the Drexel University press release explains, “This process [is] called nanostructuring [and] introduces particles, tubes, flakes and stacks of nanoscale materials as the new components of batteries, capacitors and supercapacitors. Their shape and atomic structure can speed the flow of electrons - the heartbeat of electrical energy. And their ample surface area provides more resting places for the charged particles.”
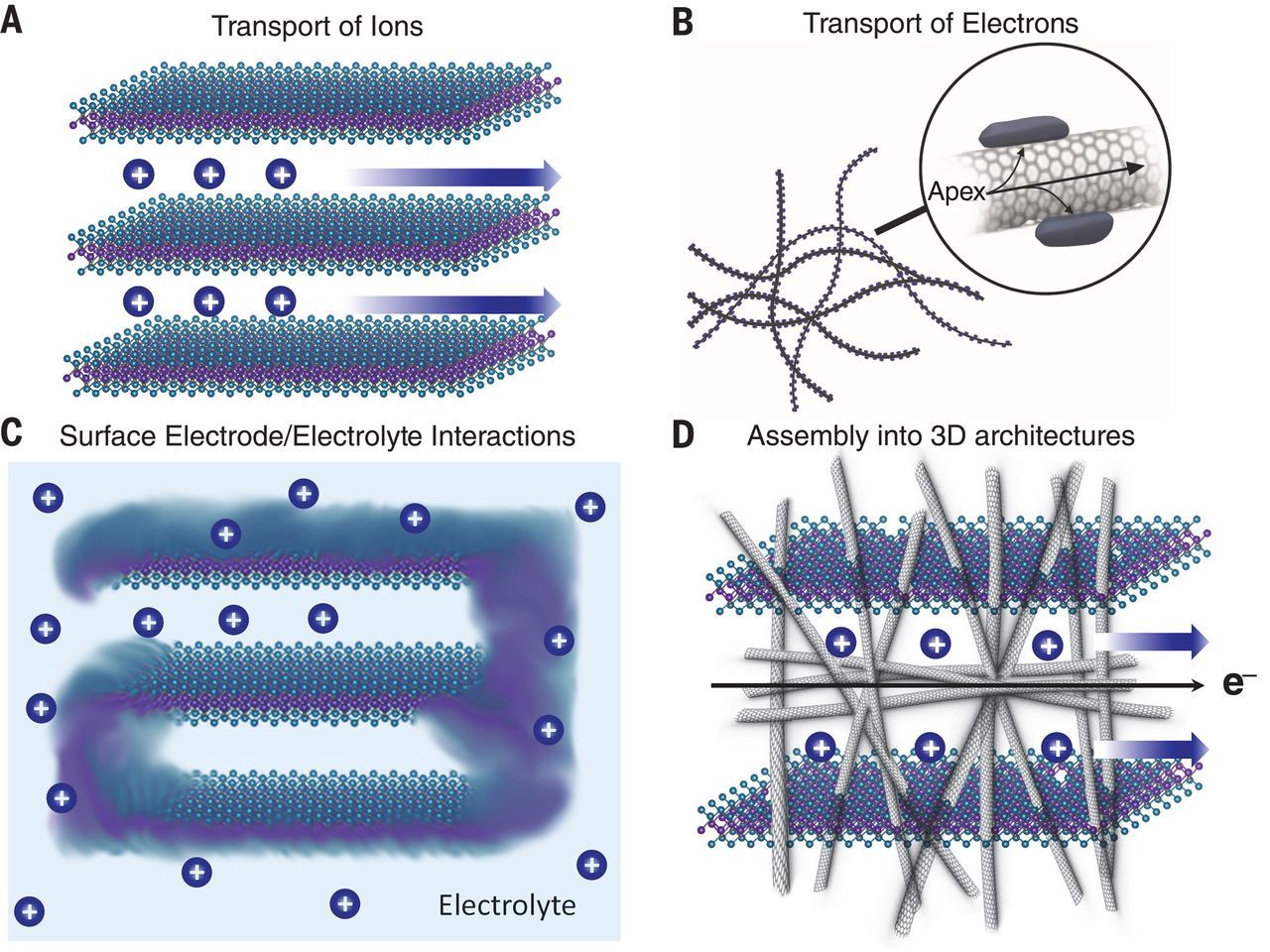
With nanomaterials at their core, scientists have been able to rethink even the most basic functions of batteries. For example, “With metallically conducting nanostructured materials ensuring that electrons can freely flow during charge and discharge, batteries can lose a good bit of weight and size by eliminating metal foil current collectors that are necessary in conventional batteries. As a result, their form is no longer a limiting factor for the devices they're powering.”
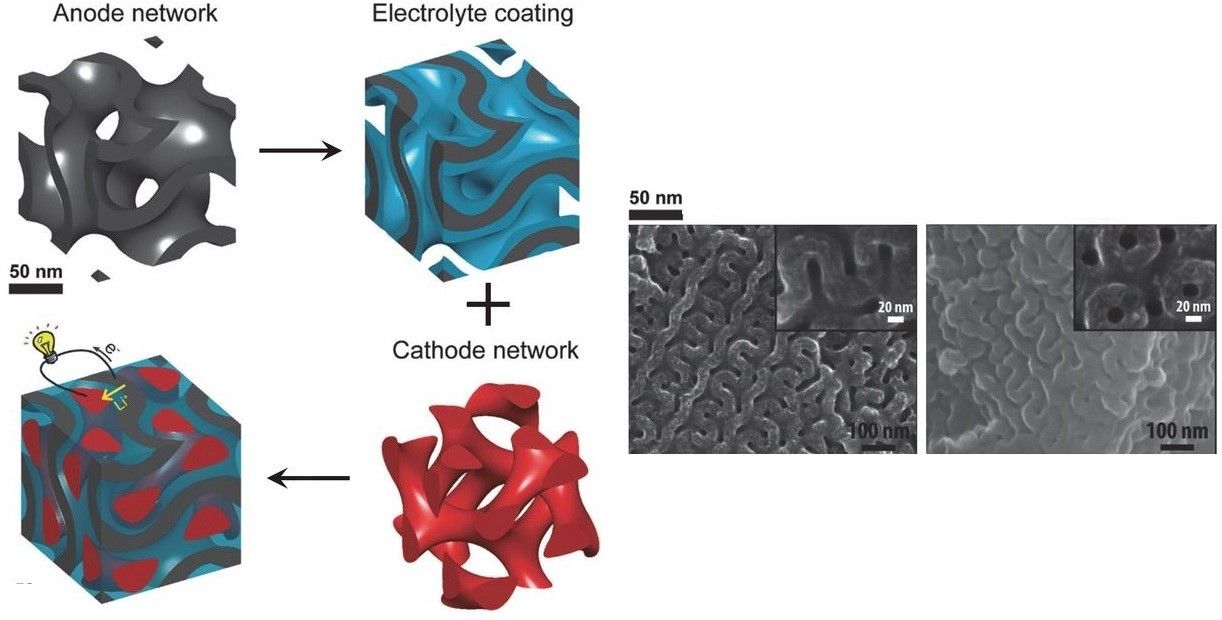
Consequently, batteries are charging faster, getting smaller, holding energy for longer, have greater capacity, and are more durable than ever before. To solve the problems of climate change requires transferring this technology to a larger scale. Batteries that can charge progressively, can safely and efficiently store energy for days, and can hold enough electricity to power a city.
“It is a very exciting time to work in the area of nanoscale energy storage materials,” said Ekaterina Pomerantseva, PhD, an associate professor in the College of Engineering and one of the paper’s co-authors. “We now have more nanoparticles available than ever - and with different compositions, shapes and well-known properties. These nanoparticles are just like Lego blocks, and they need to be put together in a smart way to produce an innovative structure with performance superior of any current energy storage device. What makes this task even more captivating is the fact that unlike Legos, it is not always clear how different nanoparticles can be combined to create stable architectures. And as these desired nanoscale architectures become more and more advanced, this task becomes more and more challenging, triggering the critical thinking and creativity of scientists.”
This means that making the break from a high-performing solar powered smartwatch battery to one that can replace a nuclear power station will not be easy. Part of making the change will be in ensuring the stability of nanomaterials when used on such an industrial scale.
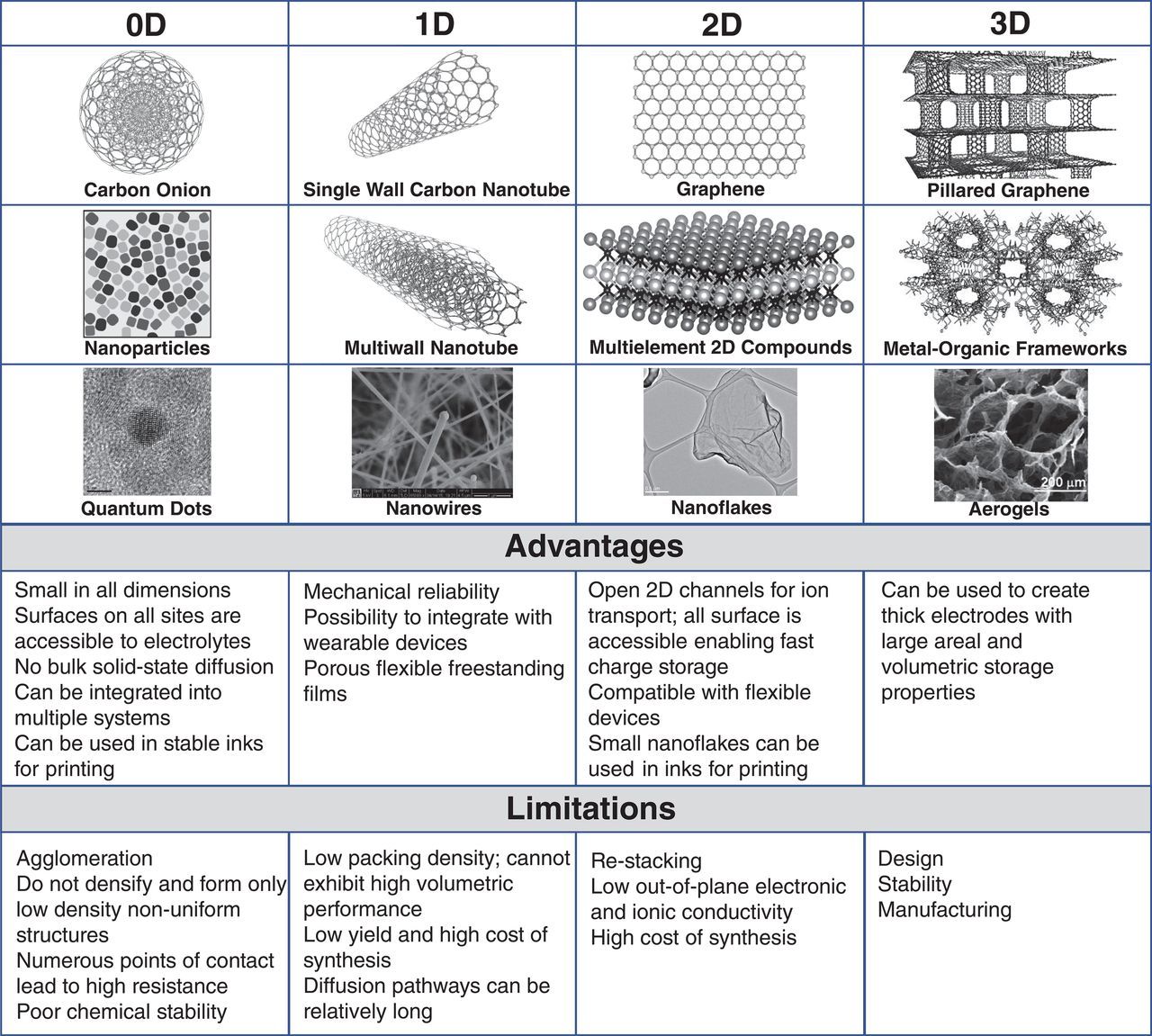
However, the biggest challenge may lie in current production methods, which have put a high price on even small amounts of nanomaterials.
As Gogotsi acknowledges, “The cost of nanomaterials compared to conventional materials is a major obstacle, and low-cost and large-scale manufacturing techniques are needed.” However, he also believes that the lowering of costs and the upscaling of nanomaterial production to the levels required is possible.

As he observes, “[Economic mass production] has already been accomplished for carbon nanotubes, with hundreds of tons of manufacturing for the needs of the battery industry in China. Pre-processing the nanomaterials in this way would allow the use of current battery manufacturing equipment.”
What can be achieved with nanotechnology for the consumer electronics sector is evident. What is required to combat climate change is clear. All that is now needed is sufficient investment to bridge the gap towards effective industrial scale energy storage.
If we want a world run on renewable energy, then we need to employ nanomaterials.
The study, entitled ‘Energy storage: The future enabled by nanomaterials’, has now been published in the journal Science.
Photo credit: Science, Climatecentral, Physicsworld, & PVBuzz
